FDA Issues EUA for Point-of-Care COVID-19 PCR Test

Advancing Visby Medical’s PCR test, a new EUA for the “personal” PCR test expands its use to patient care settings operating under certain certificates.
New Research Identifies Which Point-of-Care COVID-19 Tests Meet WHO’s Target Product Profiles
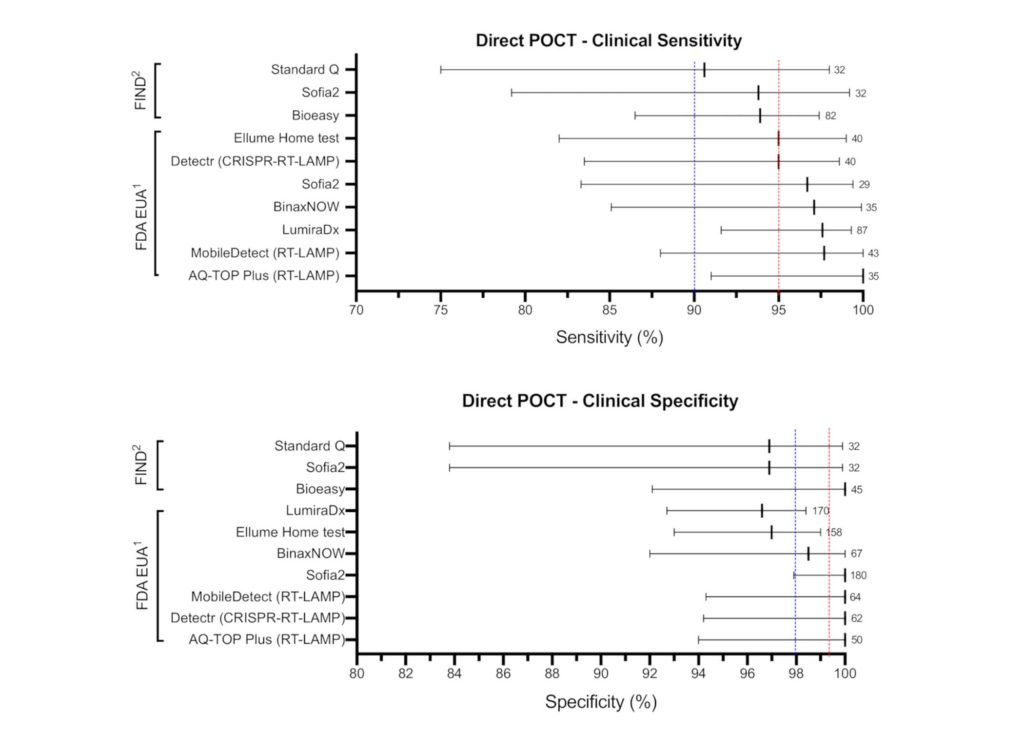
Findings of new research that focuses on the performance of rapid COVID-19 antigen and antibody tests.

