7 Highlights of the FDA EUA Policy Issued May 11

Major highlights of the FDA’s revised COVID-19 testing policy issued May 11, 2020.
FDA Acknowledges Tradeoffs of Early SARS-CoV-2 Serological Tests

In mid-March, it was critical to provide regulatory flexibility for serology test developers. However, two major issues have surged through public and political discourse.
Webinar with Westgards Will Help Your Clinical Laboratory Choose the Right Strategy Around the Coming Wave of Serology Testing
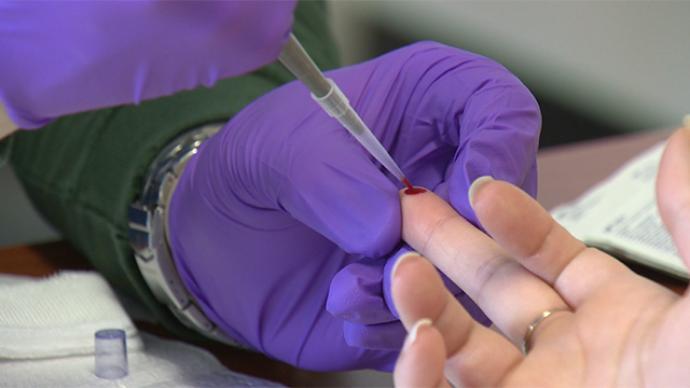
There’s good news coming for clinical laboratories as a tidal wave of orders for COVID-19 serology tests head their way while healthcare policymakers call upon tens of millions of Americans to be tested in the coming weeks and months for the COVID-19 antibodies. This will be the single largest medical testing program ever undertaken in […]

