
CDC Releases Clinical Laboratory Test Recommendations for Long-Haul COVID-19 Symptoms
A breakout of the CDC’s new focus on relatively routine clinical laboratory tests and four specialized lab tests based on a patient’s particular complaints.
COVID-19 Business Intelligence and Analysis for Clinical Laboratories, Pathology Groups and Hospital Administration
Reliable COVID-19 Business Intelligence and analysis for clinical laboratories, pathology groups and Laboratory Diagnostics.

A breakout of the CDC’s new focus on relatively routine clinical laboratory tests and four specialized lab tests based on a patient’s particular complaints.

New online CDC tool intended to relieve confusion around when to test now, as well as enable healthcare providers to quickly identify patients who should be tested for COVID-19.

The FDA cited multiple reasons for issuing the warning and recall, including the fact that the test’s performance was not adequately established.

New FDA warnings for unauthorized COVID-19 tests demonstrates the FDA’s continued attention to unreliable COVID-19 testing.

SARS-CoV-2 variants continue to create new impacts on some COVID-19 tests, FDA warns.
New COVID-19 funding announced by HHS to reimburse healthcare providers for COVID-19 testing for the uninsured.

Despite considerable and growing interest in COVID-19 antibody testing for vaccinated individuals, the FDA’s new guidance seems to exclude such testing.

Toward possibly more accurate COVID-19 test results, FDA issues new resources, reminder to healthcare providers teaching COVID-19 testing self-collection.

After months of debate, CDC makes decision on the concept of airborne transmission of COVID-19 and risk

New FDA authorization helps make COVID-19 vaccination available for children as young as 12 years old.
New OSHA regulations treat COVID-19 vaccination adverse reactions as work-related injuries when employers make vaccination mandatory.

Oregon, one of 28 states that have state Occupational Safety and Health Administration plans, is the first state to step up COVID-19 spread mitigation rules.

It remains unclear if this pause will have an effect on people’s perception of the safety of the vaccine and if it will impact vaccination rates.

What clinical laboratory leaders can expect to find.

Three new recommendations for respirator supply management.

Streamlining authorizations will allow growth of asymptomatic testing as part of school, workplace, community, and other COVID-19 testing programs.

EUA revocation indicates that SARS-CoV-2 mutations are beginning to impact COVID-19 treatment efficacies.

Johnson and Johnson COVID-19 vaccine suspended as rare blood clotting disorder appears to be associated with the vaccine.

This COVID-19 antibody test allows consumers to collect blood using a finger stick, then send the dried sample for laboratory analysis.

This new government contract emphasizes a continued interest in expanding COVID-19 testing capabilities in the US, even as the vaccine rollout is well underway and cases are declining in most locations.

New FDA webpage provides new information about how SARS-CoV-2 variants impact COVID-19 testing and how clinical laboratories can respond.

Three new authorizations granted by the FDA for over-the-counter and point-of-care COVID-19 tests used for serial testing.

The FDA has taken steps to streamline the path for COVID-19 screening tools, providing information to help groups establish mass COVID-19 testing programs.

The FDA says data was reviewed from a clinical study of more than 500 test samples and a variety of analytical studies, which demonstrated a reasonable assurance that this COVID-19 test was safe and effective at identification and differentiation of various respiratory viral and bacterial pathogens.

Next-generation sequencing (NGS)-based test reportedly diagnoses the presence of COVID-19 and also provides information about viral variants.

The clinical performance of this innovative device was studied in hospital and school settings.

In a new first, molecular testing for COVID-19 can now be performed at home without a prescription, changing the COVID-19 testing market.

This alert identifies two issues and issues three recommendations.

Centers for Medicare and Medicaid Services (CMS) makes at least eight key points about commercial payer coverage related to COVID-19 testing and vaccination.

While T-cell immunity is still a very active field of COVID-19 study, this new test now allows clinicians to offer T-cell COVID-19 testing for their patients.

New storage requirement for Pfizer vaccine makes it easier for clinical laboratory personnel to access vaccine.

Toward the performance of diagnostic tests, three main components are addressed by this new guidance.

Notice of complaint filed: TaqPath COVID-19 Combo Kit

New federal funding for pandemic response moves in three directions.

Advancing Visby Medical’s PCR test, a new EUA for the “personal” PCR test expands its use to patient care settings operating under certain certificates.

Clinical laboratories will be most affected by the FDA’s update on diagnostics.

Since the original emergency use authorization was issued in August 2020, new data has prompted this change to convalescent plasma use.

Key highlights of H.R.7663 impact on telehealth regulations.

Travel within the United States may require negative COVID-19 result if CDC implements contemplated changes.

Changes to US Food and Drug Administration’s (FDA) Discontinuance List makes reporting more useful for clinical laboratories.

Creation of COVID-19 Pandemic Testing Board may affect clinical laboratories and lead to a change in national COVID-19 testing.

Thirty-one pages of new CMS guidance distilled into 10 probing questions revealing what CMS surveyors will be looking for in clinical laboratories, with regard to their COVID-19 testing tracing and reporting.

Summary of new Centers for Disease Control and Prevention (CDC) order affecting COVID-19 testing for all passengers from foreign countries.

Curative SARS-Cov-2 diagnosis test providing high number of false negatives, FDA warns.

A new SARS-CoV-2 variant, B.1.1.7, was identified late last year in the UK, prompting the FDA to monitor existing tests, such as TaqPath, Linea, and Accula.

US Centers for Disease Control and Prevention orders airlines to verify and confirm documentation for COVID-19 negative testing for certain travelers.

Six questions pertaining to the US Food and Drug Administration’s updated medical Device Shortage List, and the primary testing supplies that were added.

“Through the FDA’s open and transparent scientific review process, …” : FDA Commissioner Stephen M. Hahn, MD, about the second EUA to cover a COVID-19 vaccine.

The guidance explains when clinical laboratories can begin testing for SARS-CoV-2 after submitting the CMS-116 application for a CLIA Certificate of Waiver.

Guidance changed for three questions about CLIA standards pertaining to COVID-19 testing.

The FDA continues to accelerate at-home COVID-19 test accessibility for consumers with this, the third emergency use authorization for a fully at-home test.

Insight into the federal payments that clinical laboratories received after CMS expanded its Accelerated and Advance Payment (AAP) Program to increase cash flow to Medicare providers and suppliers impacted by the 2019 novel coronavirus (COVID-19) public health emergency.

Explanation of the range of new guidance for approaching COVID-19 testing in nursing homes.
Convalescent plasma titers can now be qualified using the Mount Sinai COVID-19 ELISA IgG Antibody Test.

New guidance on what should be submitted to the FDA in support of an EUA request or pre-EUA request for a SARS-CoV-2 antibody test.

Recent developments in emergency use authorizations for investigational therapies for use in the treatment of mild to moderate COVID-19.

Points of interest from the US Food and Drug Administration’s November 2020 update to investigational COVID-19 convalescent plasma guidelines.

More about the first at-home COVID-19 test, a rapid, single-use molecular diagnostic test kit for the qualitative detection of SARS-CoV-2 RNA from self-collected nasal swab specimens.

Tests of interest as part of new FDA-allowed at-home specimen collection options.

Breakout of examples of modifications that will and will not be permitted with influenza and RSV clinical lab tests during the public health emergency.

With over 50 COVID-19 antibody tests authorized for use under FDA EUA, a milestone has been recognized with the FDA’s Nov. 6, 2020, announcement.

Key update and recommendations released Oct. 26, 2020, that affect SARS-CoV-2 antigen test use.

October 2020 Medicare loan repayment update for providers.

New FDA update to comparative COVID-19 test performance data now includes information about most available SARS-CoV-2 diagnostic molecular tests.

Interim final rule (CMS-3401-IFC) from CMS mandates daily reporting for broader surveillance of COVID-19 and influenza statistics, and outlines strict enforcement mechanisms.

Rollout and availability of rapid SARS-CoV-2 antigen tests opens wave of CLIA certification issues triggering CMS cease and desist letters.

Three teaching points for self-collecting SARS-CoV-2, COVID-19 specimens; FDA cautions healthcare providers about poor specimen quality.

Quick links to clarification of CMS policy for clinical laboratories and updated FDA recommendations to healthcare providers about antigen testing of certain asymptomatic individuals.

A six-point primer to the new CMS clinical laboratory CLIA quick start guide released in September 2020.

With 47 COVID-19 antibody tests authorized for use under FDA EUA as of Sept. 24, 2020, only one is authorized as POC for fingerstick blood samples collected in patient care settings.

Lowest limit of detection noted.

Three essential points pertaining to university laboratories planning for SARS-CoV-2 surveillance testing.

Guidance released Sept. 8 provides simplified details on nursing home testing that should be performed, also identifies three circumstances related to COVID-19 testing triggers.

Four key points from the FDA’s September 2020 update to initial COVID-19 convalescent plasma guidelines.

Combination coronavirus flu tests readying for flu season.

Details fall into place for invoking the Defense Production Act for SARS-CoV-2 antigen test production.

Points of interest from CMS-3401-IFC and CLIA requirements modified in August 2020.

New guidance for those seeking approval or clearance of, or an EUA for, an LDT, and for those opting to use LDTs in their laboratories without FDA premarket review or authorization.

The four companies implicated in recent FDA warning letters over unauthorized COVID-19 tests. What did these tests have in common?

Update on emergency use authorization for third SARS-CoV-2 POC antigen test.
What the National Cancer Institute (NCI) found toward the revocation of Autobio Diagnostics’ antibody test EUA.

New developments in measuring SARS-CoV-2 antibodies.

Overview of new CMS resource for understanding SARS-CoV-2 antigen test distribution to nursing homes.

FDA guidance provides pathway for developers and manufacturers to obtain EUAs for at-home and over-the-counter COVID-19 testing. The US Food and Drug Administration (FDA) issued

New EUA opens the door for increased availability of COVID-19 testing for asymptomatic individuals.

The FDA recently updated its interactive tool designed to assist in identifying COVID-19 testing supply alternatives.

Shipping guidelines for SARS-CoV-2 specimens emphasized as many clinical laboratories are not shipping specimens correctly.

Pooled SARS-CoV-2 diagnostic testing is now available after FDA issues first EUA for this testing method.
A look at new COVID-19 test data reporting requirements for in-house clinical laboratories of critical access hospitals, children’s hospitals, general hospitals (including acute, trauma, and teaching hospitals), long-term acute care hospitals, military hospitals, oncology hospitals, orthopedic hospitals, pediatric long-term acute care hospitals, psychiatric hospitals, rehabilitation hospitals, surgical hospitals, Veterans Administration hospitals, women’s hospitals, and women’s and children’s hospitals, according to HHS.

Overview of detection detailed in combination SARS-CoV-2 and flu testing EUAs.

Interest is increasing in pooled sampling for SARS-CoV-2 surveillance testing. CMS addressed three primary questions in a recent FAQ.

Five points of interest to clinical laboratory leaders, gleaned from an over 8,000-word payer guidance document jointly developed by federal agencies.

This point-of-care test can potentially scale up to test millions, according to FDA Commissioner Stephen Hahn, MD.

Explanation of warnings issued to KBMO Diagnostics related to at-home COVID-19 testing.

Where to start when working on a laboratory developed test using next generation sequencing (NGS) technology for diagnosing SARS-CoV-2, according to Timothy Stenzel, MD, PhD.

Selected quotes from Jeffrey Shuren, MD, JD, Director of the Center for Devices and Radiological Health at the FDA on the June 16, 2020, updates to EUA templates.

Four important statements from a June 8 CDC clinical laboratory call covering COVID-19 test data reporting requirements.

As of June 12, 2020, the FDA had authorized 135 COVID-19 tests under emergency use authorization (EUA), which include 114 molecular tests, 20 antibody tests, and 1 antigen test. The FDA has also released a new interactive tool designed to assist in identifying COVID-19 testing supply deficiencies and alternatives.

The FDA addresses concerns of unreliable tests by providing a new tool to support their evaluation of SARS-CoV-2 diagnostic tests.

SARS-CoV-2 test reporting requirements just multiplied. Clinical laboratories are on a deadline now.
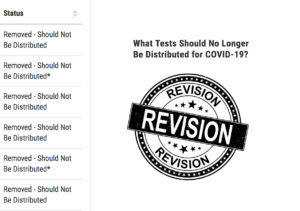
Introduction to commercially manufactured SARS-CoV-2 serology tests listed with the status of “Removed – Should Not Be Distributed.”

While not specifically addressed by CMS, the U.S. Department of Health and Human Services (HHS) may fine non-compliant institutions up to $300 per day.

Multiple-step methods versus single-step methods differentiated.

As the number of COVID-19 cases in the United States has increased, the availability of real COVID-19 positive specimens has also increased.

Segmented highlights from interim final rule CMS-5531-IFC, which was released with a 60-day comment period, effective May 8, 2020. Recent changes are intended to increase the COVID-19 response capabilities of providers and hospitals.

Major highlights of the FDA’s revised COVID-19 testing policy issued May 11, 2020.

Compare SARS-CoV-2 testing requirements for clinical laboratories and other facilities based on new 20-06-CLIA memo issued April 30, 2020.

Clinical laboratory directors may reduce their risk of fraud and waste by familiarizing themselves with these four areas of recent focus.

Impact of relaxing HIPAA enforcement during COVID-19 pandemic.

A breakout of the CDC’s new focus on relatively routine clinical laboratory tests and four specialized lab tests based on a patient’s particular complaints.

New online CDC tool intended to relieve confusion around when to test now, as well as enable healthcare providers to quickly identify patients who should be tested for COVID-19.

The FDA cited multiple reasons for issuing the warning and recall, including the fact that the test’s performance was not adequately established.

New FDA warnings for unauthorized COVID-19 tests demonstrates the FDA’s continued attention to unreliable COVID-19 testing.

SARS-CoV-2 variants continue to create new impacts on some COVID-19 tests, FDA warns.

New COVID-19 funding announced by HHS to reimburse healthcare providers for COVID-19 testing for the uninsured.

A breakout of the CDC’s new focus on relatively routine clinical laboratory tests and four specialized lab tests based on a patient’s particular complaints.

New online CDC tool intended to relieve confusion around when to test now, as well as enable healthcare providers to quickly identify patients who should be tested for COVID-19.

The FDA cited multiple reasons for issuing the warning and recall, including the fact that the test’s performance was not adequately established.

New FDA warnings for unauthorized COVID-19 tests demonstrates the FDA’s continued attention to unreliable COVID-19 testing.

SARS-CoV-2 variants continue to create new impacts on some COVID-19 tests, FDA warns.

New COVID-19 funding announced by HHS to reimburse healthcare providers for COVID-19 testing for the uninsured.