3 Frequently Asked Questions About COVID-19 Price Transparency Requirements

While not specifically addressed by CMS, the U.S. Department of Health and Human Services (HHS) may fine non-compliant institutions up to $300 per day.
CRISPR Technology in SARS-CoV-2 Diagnostic Testing May Provide Clinical Laboratories With More Cost Effective, Faster Testing Method
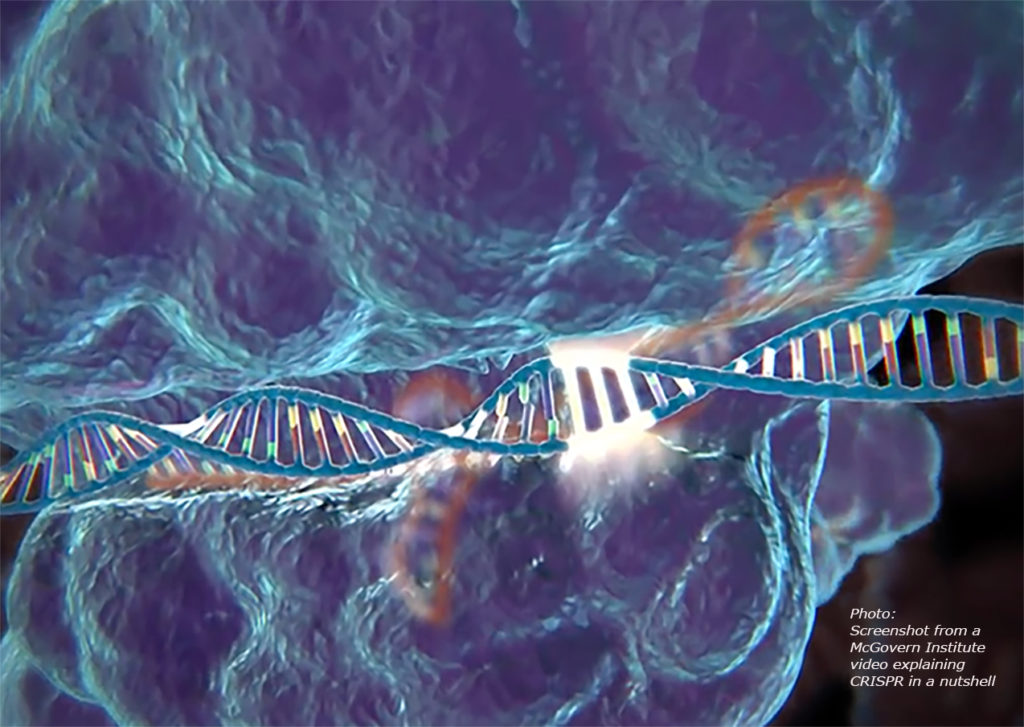
With this recently approved testing paradigm that has emerged within the last few weeks, a significant question remains.

