FDA Reissues EUA for Convalescent Plasma to Permit the Use of Additional Antibody Test
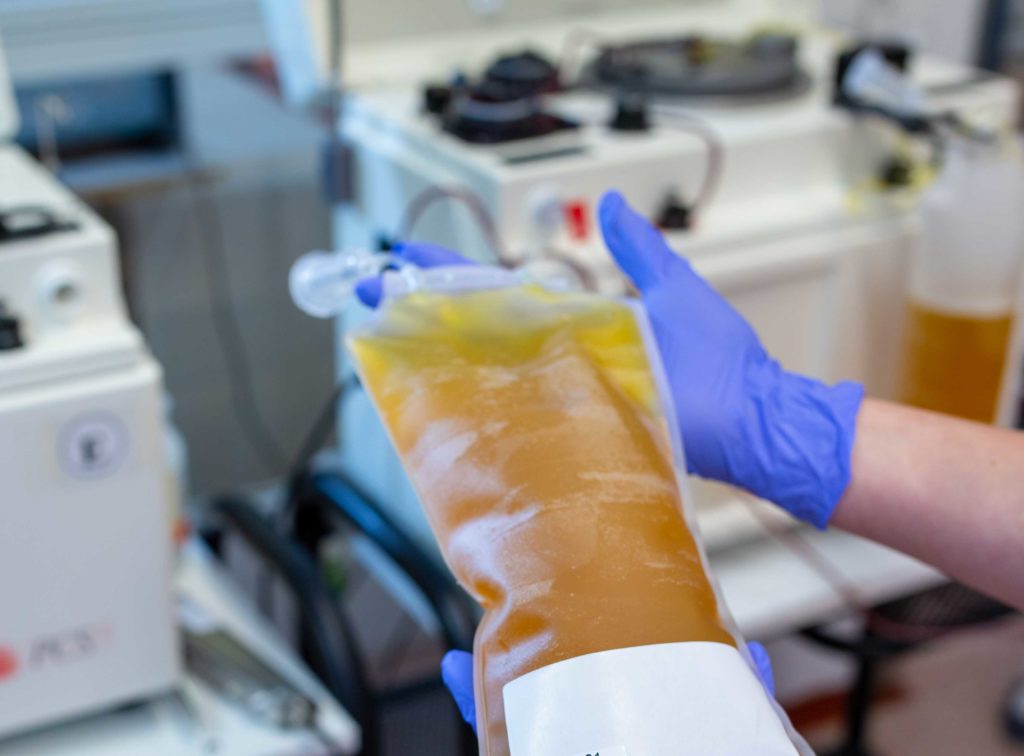
Convalescent plasma titers can now be qualified using the Mount Sinai COVID-19 ELISA IgG Antibody Test.
Recent Study Concludes That No Routine Clinical Laboratory Test Reliably Serves as a Biomarker for COVID-19

More on a recent study of 67 routine clinical laboratory tests that sought to identify COVID-19 biomarkers.

