FDA Releases New EUA Template for COVID-19 Screening Tests that Involve Serial Testing

The FDA has taken steps to streamline the path for COVID-19 screening tools, providing information to help groups establish mass COVID-19 testing programs.
New Assay Allows for Customizable Testing of SARS-CoV-2 Variants Using PCR Technology
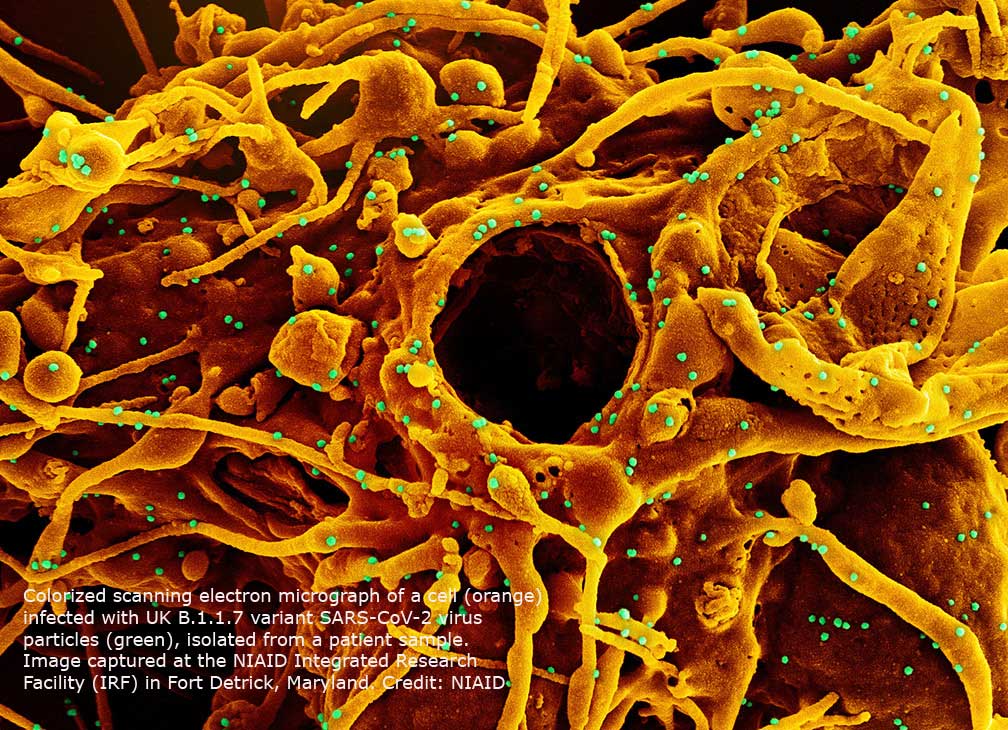
The emergence of multiple SARS-CoV-2 variants brings fast-moving questions for clinical laboratory administrators about the ability to identify these new variants. A new mutation panel may offer an alternative to viral genome sequencing for indicating the possible presence of these variants.

