Federal Government Awards $53.7 Million Deal to Increase COVID-19 Test Production

This new government contract emphasizes a continued interest in expanding COVID-19 testing capabilities in the US, even as the vaccine rollout is well underway and cases are declining in most locations.
FDA Publishes New Webpage That Consolidates Information About How SARS-CoV-2 Variants Impact COVID-19 Tests

New FDA webpage provides new information about how SARS-CoV-2 variants impact COVID-19 testing and how clinical laboratories can respond.
In Bid to Increase Serial Testing Capabilities, FDA Significantly Expands Authorization for Over-the-Counter COVID-19 Tests

Three new authorizations granted by the FDA for over-the-counter and point-of-care COVID-19 tests used for serial testing.
Vaccine Breakthrough Provides Process That Produces $1 Vaccines in Less Than Three Weeks of Development

As new vaccine breakthrough research continues, Virginia Tech and UVA virologists develop innovative COVID-19 vaccine production platform.
FDA Releases New EUA Template for COVID-19 Screening Tests that Involve Serial Testing

The FDA has taken steps to streamline the path for COVID-19 screening tools, providing information to help groups establish mass COVID-19 testing programs.
New Assay Allows for Customizable Testing of SARS-CoV-2 Variants Using PCR Technology
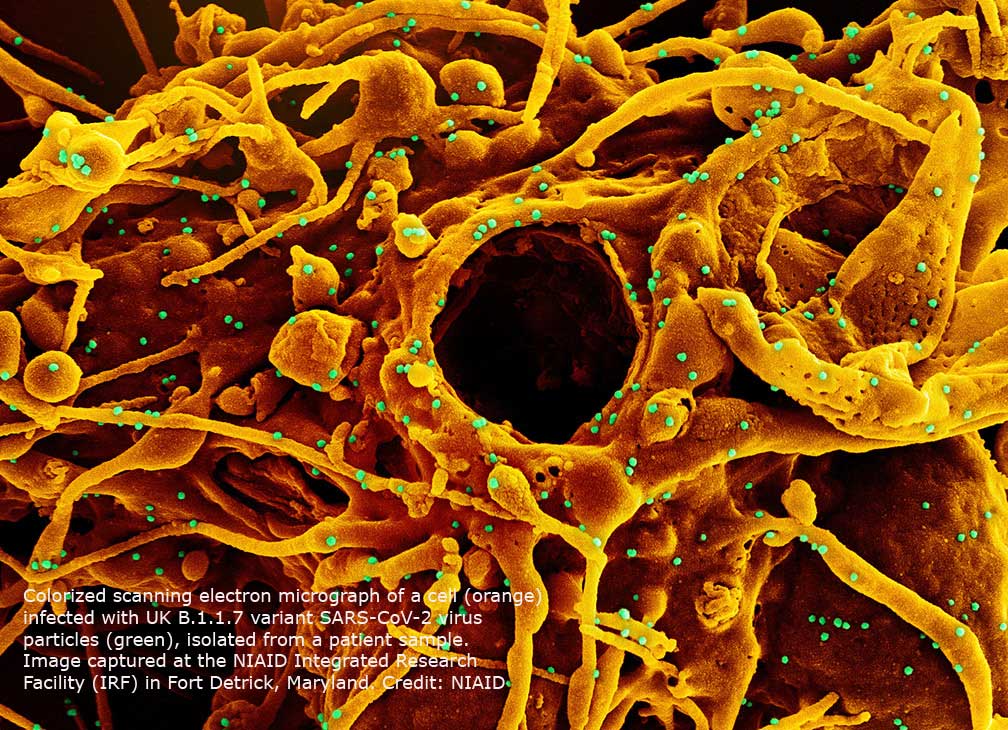
The emergence of multiple SARS-CoV-2 variants brings fast-moving questions for clinical laboratory administrators about the ability to identify these new variants. A new mutation panel may offer an alternative to viral genome sequencing for indicating the possible presence of these variants.
In a New First, FDA Permits Marketing of COVID-19 Test Through Traditional Premarket Review Process

The FDA says data was reviewed from a clinical study of more than 500 test samples and a variety of analytical studies, which demonstrated a reasonable assurance that this COVID-19 test was safe and effective at identification and differentiation of various respiratory viral and bacterial pathogens.
FDA Issues Second EUA for Whole Genome Sequencing COVID-19 Diagnostic Test

Next-generation sequencing (NGS)-based test reportedly diagnoses the presence of COVID-19 and also provides information about viral variants.
FDA Issues EUA for First Machine Learning Device That Screens for COVID-19

The clinical performance of this innovative device was studied in hospital and school settings.
COVID-19 Test Results May Vary Based on Time of Day Specimens Were Collected, New Study Shows

The new research indicating a time-dependent result for COVID-19 testing may have significant implications for the clinical laboratories.

