Interest continues to grow in the use of convalescent plasma to treat COVID-19 patients, and clinical laboratories in many areas are helping in this effort.
Convalescent plasma is a novel COVID-19 treatment that is currently undergoing studies and may become more prevalent as clinicians search for methods of treating those infected with COVID-19. To better understand the current state of this potential treatment option and the implications that it could have for clinical laboratories, the COVID-19 STAT Intelligence Briefings Service spoke with an investigator working in clinical trials being run by the Mayo Clinic.
Elitza S. Theel, PhD, is an Associate Professor of Laboratory Medicine and Pathology at Mayo Clinic in Rochester, Minnesota. Theel’s work focuses on the development of new serologic assays and the evaluation of currently available serologic testing methods to aid in the diagnosis of a variety of bacterial, viral, and fungal infectious diseases. Along with Principal Investigator Michael J. Joyner, MD, Theel is working on convalescent plasma therapy clinical trials led by Mayo Clinic in collaboration with government, industry, and academic partners.

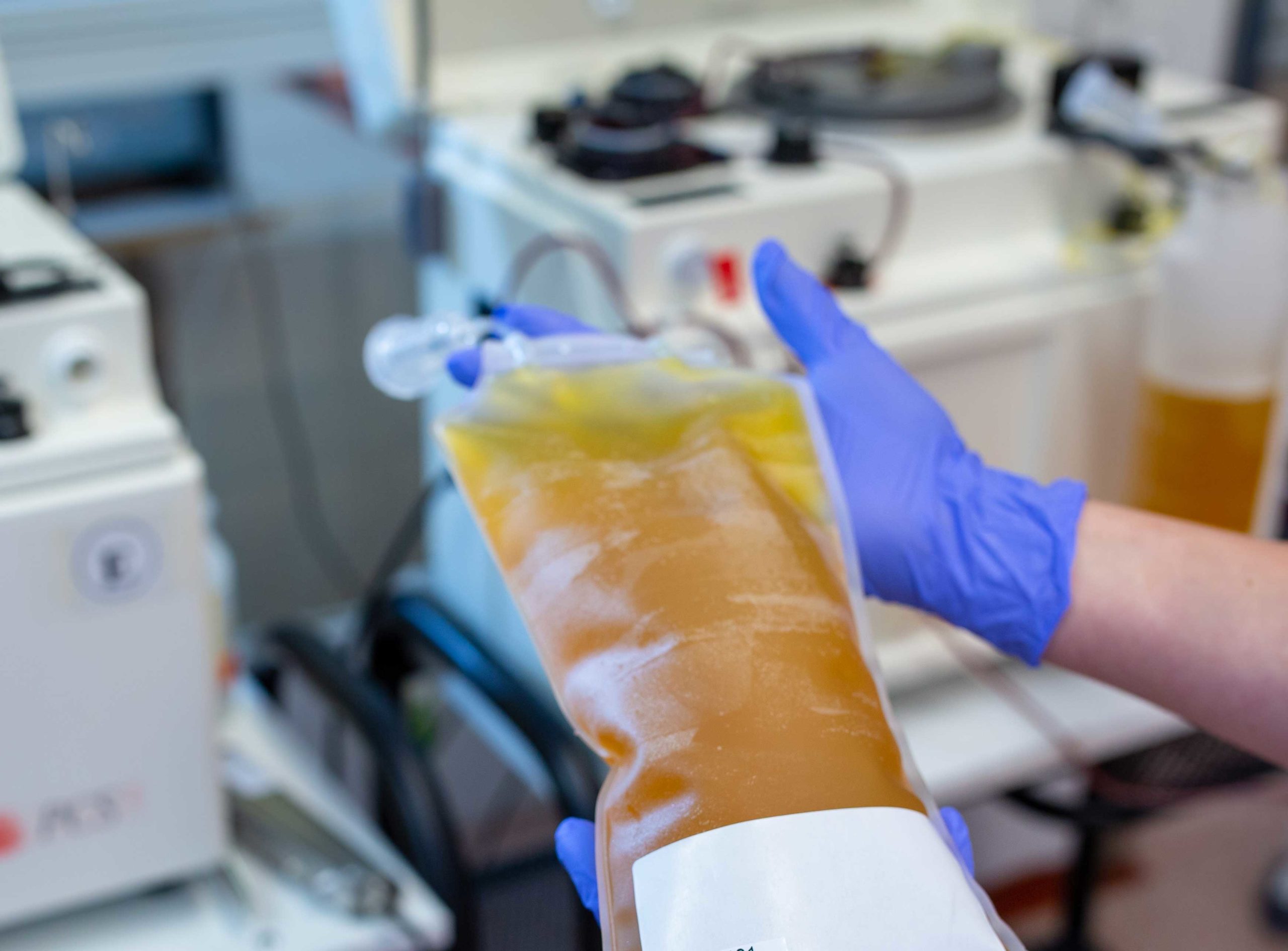
For the COVID-19 STAT Intelligence Briefings Service, Elitza S. Theel, PhD, of the Mayo Clinic in Rochester, Minnesota, outlined progress in convalescent plasma therapy as an experimental treatment for COVID-19. Convalescent plasma therapy, while not approved by the US Food and Drug Administration (FDA), has been greenlighted with special protocols as an investigational product for treating COVID-19, with efforts led by Mayo Clinic. Many clinical laboratories in hospitals and health systems across the country are helping with convalescent plasma therapy programs. (Photo used with permission.)
Basic Principles of Convalescent Plasma in COVID-19 Treatment
Theel explained the basic principles of how convalescent plasma is being used. “The concept is to take plasma from a person that has had COVID-19 and has recovered,” Theel explained. “Plasma is then isolated from the blood donation and once it is confirmed that there are antibodies to COVID-19, the plasma is transfused into an individual that is currently sick with COVID-19. The idea being those antibodies would bind and inactivate the SARS-CoV-2 virus.”
While, in theory, convalescent plasma could be used to create immunity, this is not the primary purpose being pursued by current clinical trials. “Questions remain about whether plasma like this can be used preemptively to provide passive immunity to high-risk individuals; that, too, is being pursued as a clinical trial-type study,” Theel said. “That use of convalescent plasma may be under evaluation in some clinical settings.”
Recent COVID-19 Convalescent Plasma Guidance and Results
The US Food and Drug Administration issued guidance May 1, 2020, as part of its blood and biologics, investigational new drug (IND) or device exemption (IDE) resources. This guidance explains that COVID-19 convalescent plasma is collected from individuals who meet certain qualifications:
- Evidence of COVID-19 documented by a laboratory test either by a diagnostic test (e.g., nasopharyngeal swab) at the time of illness; or
- A positive serological test for SARS-CoV-2 antibodies after recovery, if prior diagnostic testing was not performed at the time COVID-19 was suspected.
The FDA also specifies that there must be complete resolution of symptoms at least 14 days before the donation, and a negative result for COVID-19 by a diagnostic test is not necessary to qualify the donor.
Additionally, COVID-19 convalescent plasma has not yet been approved for use by FDA; therefore, it is regulated as an investigational product, according to the FDA’s May 1 guidance.
Results occurring with convalescent plasma treatments have not been published but are likely to become available soon, Theel said. “Anecdotally, I would say that there are promising findings,” she added. “There are many publications that are in the works. One question that’s out there is what level of antibodies—specifically neutralizing antibodies—are important for the plasma that’s being donated to have for it to be effective in the individuals. Hopefully, we’ll have data on that in the coming weeks.”
As of July 6, the convalescent plasma COVID-19 Expanded Access Program (EAP), led by Mayo Clinic, involved 2,547 sites, 9,971 physicians, 48,125 patients, with 31,497 infused. Moreover, a nationwide campaign for plasma donation runs in parallel.
For Mayo Clinic News, Robert Nellis describes two COVID-19 treatment approaches that urgently require collection of convalescent plasma from survivors. One is direct transfusion. The other is in the development of hyperimmune globulin (H-Ig) medicine that Nellis explains this way: “Through the manufacturing process, the plasma is pooled, concentrated and purified, resulting in a vial of medicine with consistent levels of antibodies that could make H-Ig easier for hospitals to store and administer to patients. Coalition members developing an H-Ig include the CoVIg-19 Plasma Alliance (CSL Behring, Takeda, ADMA Biologics, Biopharma Plasma, Biotest, BPL, GC Pharma, LFB, Octapharma and Sanquin), and Grifols.”
Opportunities for Specialized Clinical Laboratory Testing
Looking forward, clinical laboratories will play an important role in identifying donors and performing specialized testing relating to convalescent plasma. According to Theel, at Mayo Clinic there are two levels of testing to identify potential donors. Initially, more routine SARS-CoV-2 serology testing is performed to identify potential candidates. “This approach is useful because we can quickly screen donors and say right off the bat if they’re antibody negative,” Theel explained. “In that case, you probably wouldn’t want to enroll them in a COVID-19 plasma donor study.”
Once a potential donor has been identified as antibody positive for SARS-CoV-2, additional testing is needed to detect neutralizing antibodies. “Detecting those neutralizing antibodies is very challenging,” Theel told STAT COVID-19, adding that Mayo is working in collaboration with a commercial partner to detect these neutralizing antibodies. “So, moving forward, we’ll be able to say ‘yes, this donor does have antibodies, including neutralizing antibodies, and they have this level of neutralizing antibodies’.”
Clinical laboratory testing may also be needed for recipients of convalescent plasma treatments. While Theel was not able to provide specifics, she told STAT COVID-19, “Any donor would be screened routinely for other blood-borne pathogens and typing.”
For more insights into jumpstarting a convalescent plasma program, COVID-19 STAT hosted a live call June 25, featuring Randy German, MT(ASCP)SBB, Laboratory Administrative Director for Hoag Memorial Hospital. Access the program here.
As clinical data for convalescent plasma treatment becomes available in the upcoming weeks, it is likely this potential COVID-19 treatment option will become more widely used. Clinical laboratories should consider how this is likely to affect their testing volumes, specifically in serology testing for identifying potential donors.

—By Caleb Williams, Editor, COVID-19 STAT
Related Resources:
COVID Live Call #10: COVID-19: Convalescent Plasma Successful Partnership for Patient Treatment
Convalescent Plasma National Expanded Access Treatment Protocol Site
FDA Guidance Issued May 1, 2020
Early indicators: Investigational convalescent plasma is safe for patients with COVID-19
National campaign, big names calling on COVID-19 survivors to help others defeat it