Recent study shows which point-of-care (POC) COVID-19 testing options are considered “desirable” by WHO
The World Health Organization (WHO) releases criteria for diagnostic tests and treatment that describes the “desirable” profiles that health products should meet. These criteria are listed in a document specific to each product called a Target Product Profile (TPP).
In late September 2020, WHO released TPPs for various types of COVID-19 tests. According to WHO, “these TPPs describe the desirable and minimally acceptable profiles for four [types of] tests.” The TPPs are intended to serve as a guide for manufacturers and clinicians.
Now, a study by researchers at NSF International and Novateur Ventures has evaluated the rapid-detection COVID-19 tests, analyzing and identifying which point-of-care COVID-19 tests meet WHO’s TPPs for COVID-19 tests. The study, COVID-19 Point-of-Care Diagnostics That Satisfy Global Target Product Profiles, was published in the January 2021 issue of Diagnostics.
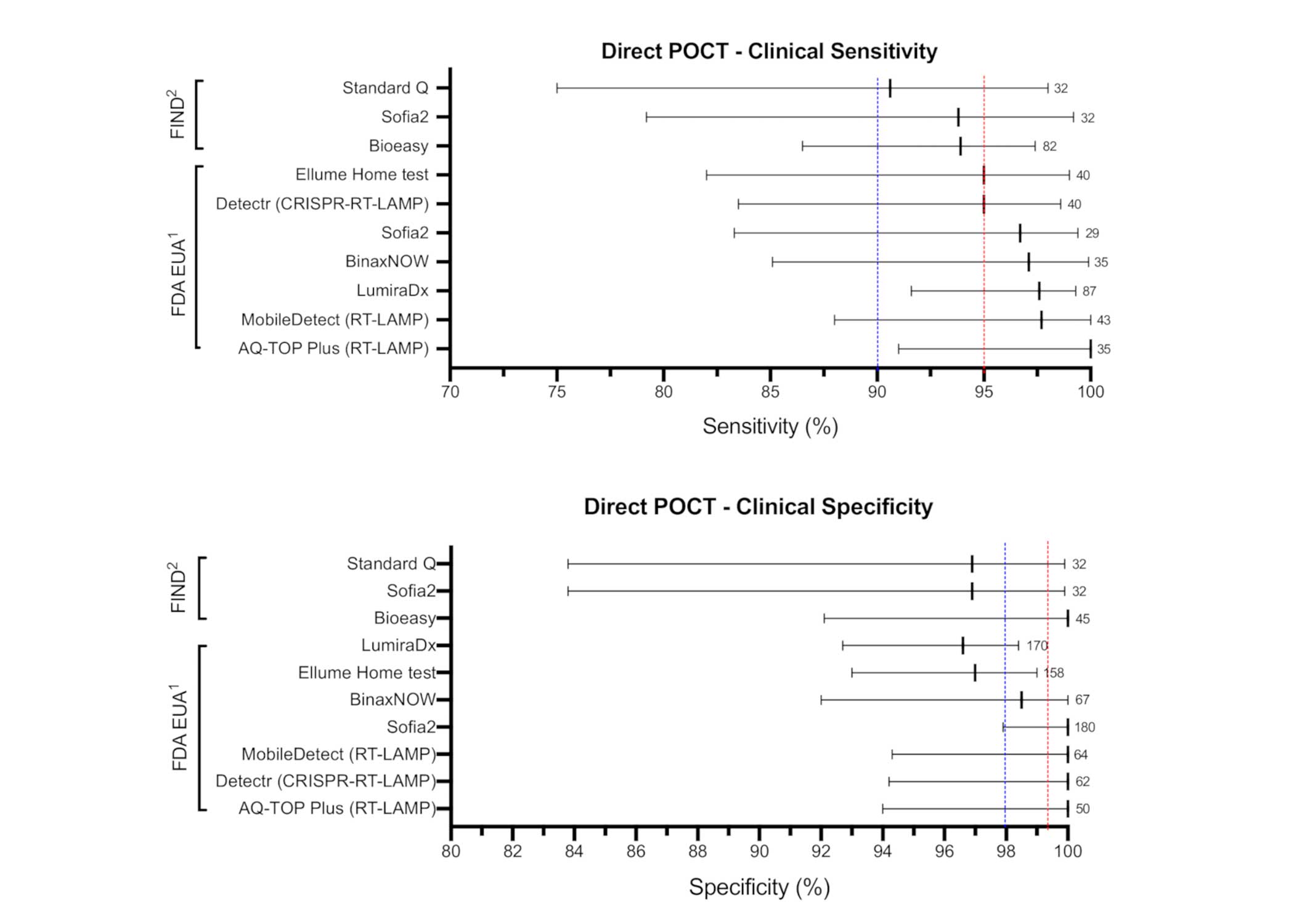
Among the research, two types of COVID-19 point-of-care (POC) tests were analyzed: direct (antigen/RNA) tests to detect acute infection and indirect (antibody) tests to detect past infection. The chart below shows a snapshot of what is reported in Diagnostics.
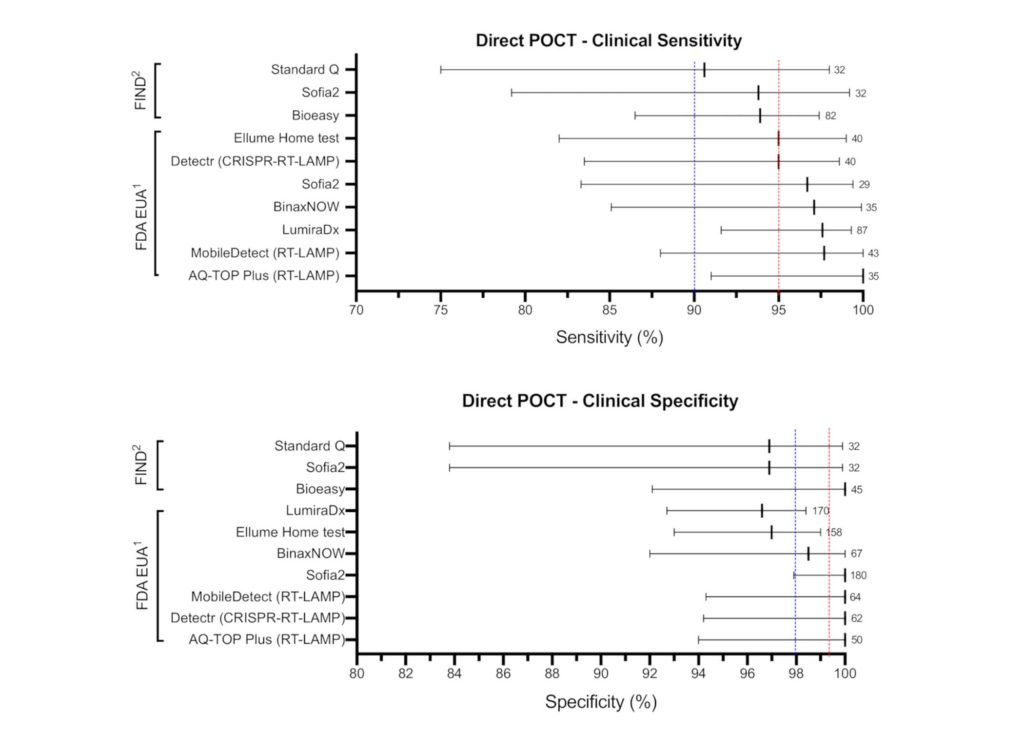
According to the study, several COVID-19 point-of-care (POC) tests met the WHO TPP “desirable” criteria for clinical sensitivity/specificity, limit of detection, and time to results.
Direct (Antigen/RNA) POC Tests Meeting WHO TPP
- DetectaChem MobileDetect Bio BCC19 Test (RT-LAMP)
- Mammoth Biosciences SARS-CoV-2 Detectr Test (RT-LAMP/CRISPR)
- Quidel Sofia-2 Flu+SARS Antigen Test
- Seasun Biomaterials AQ-TOP Plus COVID-19 Rapid Test (RT-LAMP)
- Shenzhen Bioeasy Biotechnology Bioeasy Diagnostic Kit COVID-19 Antigen Test
Indirect (Antibody) POC Tests Meeting WHO TPP
- Guangzhou Wondfo Biotech Wondfo SARS-CoV-2 Ab Test 1
- Hangzhou Biotest Biotech RightSign COVID-19 IgG/IgM Rapid Test
- Hangzhou AllTest Biotech AllTest COVID-19 IgG/IgM Rapid Test 1
- NG Biotech NG IgG/IgM Rapid Test
- Sugentech SGTi-flex COVID-19 IgG
- VivaChek Biotech COVID-19 IgM/IgG Rapid Test
“A few weeks has passed since we conducted our research in December 2020, so it is likely that additional tests have undergone independent assessment and can meet the WHO criteria now,” said Robyn Meurant, Executive Director of Health Sciences at NSF International. “The most important takeaway from this study is that purchasers of point-of-care COVID tests should do their research and make sure they are selecting a test that meets the WHO TPP criteria for their specific use case.”

—By Caleb Williams, Editor, COVID-19 STAT
Related Resources:
Diagnostics: COVID-19 Point-of-Care Diagnostics That Satisfy Global Target Product Profiles
NSF International: New Study Identifies Top-Performing Point-of-Care COVID-19 Tests