New Jersey Laboratory Sues Thermo Fisher, Alleging Problems With COVID-19 Testing Technology

Notice of complaint filed: TaqPath COVID-19 Combo Kit
Emerging SARS-CoV-2 Mutations: Impact on Thermo Fisher Scientific Assay Performance
Available for free streaming: Learn the important clinical implications (epidemiological, prognostic, and predictive) of new COVID-19 “variants of concern,” the importance of early detection of these variants, and steps your lab can take to confidently detect the COVID-19 virus with a very high degree of specificity and sensitivity, given new variants.
First Study to Ever Deliberately Infect Humans With SARS-CoV-2 Set to Provide Valuable Information About Viral Load
This new SARS-CoV-2 study carries risks and controversy.
White House Announces Over $1.6 Billion to Increase Availability of COVID-19 Testing

New federal funding for pandemic response moves in three directions.
FDA Issues EUA for Point-of-Care COVID-19 PCR Test

Advancing Visby Medical’s PCR test, a new EUA for the “personal” PCR test expands its use to patient care settings operating under certain certificates.
New Research Identifies Which Point-of-Care COVID-19 Tests Meet WHO’s Target Product Profiles
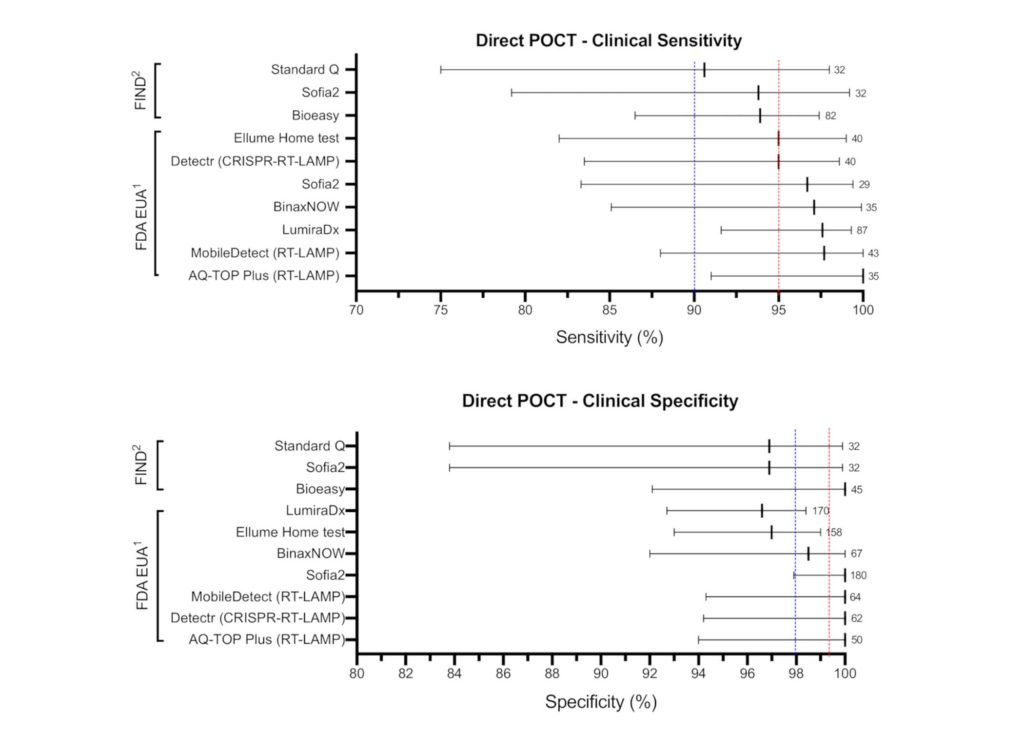
Findings of new research that focuses on the performance of rapid COVID-19 antigen and antibody tests.
Lessons One Clinical Laboratory Has Learned as It Passes Its One Millionth COVID-19 Test

Intermountain Healthcare Laboratories credit “adaptiveness” to achieving COVID-19 test milestone.
FDA Issues Update on Its Support of Medical Products Affected by SARS-CoV-2 Variants

Clinical laboratories will be most affected by the FDA’s update on diagnostics.
Dartmouth Researcher Discusses Implications New SARS-CoV-2 Variants Will Have on Clinical Laboratories

Joel Lefferts, PhD, of the Geisel School of Medicine at Dartmouth explains three major impacts that SARS-CoV-2 variants may have on testing in clinical laboratories.
FDA Tightens Requirement for COVID-19 Convalescent Plasma Testing and Use
Since the original emergency use authorization was issued in August 2020, new data has prompted this change to convalescent plasma use.

