
Long-Term COVID-19 Effects Are Caused by Brain Tissue Loss, New Research Finds
Brain imaging research would seem to indicate that the lasting effects of COVID-19 brain changes could be permanent.
COVID-19 Business Intelligence and Analysis for Clinical Laboratories, Pathology Groups and Hospital Administration
Reliable COVID-19 Business Intelligence and analysis for clinical laboratories, pathology groups and Laboratory Diagnostics.

Brain imaging research would seem to indicate that the lasting effects of COVID-19 brain changes could be permanent.

What University of Southern California research showed about suppression of cells’ mitochondria in COVID-19 immune system response.

Bitcoin becomes a potential method of payment for COVID-19 testing for point-of-care tests performed by one Florida laboratory.

Recent shift in perspective and openness to theories about SARS-CoV-2 origins mean that the truth behind the COVID-19 pandemic will eventually be discovered.

New findings about an innovative method for COVID-19 diagnostic testing that is said to provide instant results.

COVID-19 breakthrough shows that the virus uses three different techniques to evade the immune system and increase its viral load in infected individuals.

What Texas researchers found that changed baseline gene expression in airway cells.

Ultimately this research shows that the 6-foot rule should not be a core component of COVID-19 spread mitigation strategies, says MIT professor.

Clinical laboratories would test for COVID-19 antibodies in those with long-haul COVID-19 symptoms but are unsure if they were ever infected with SARS-CoV-2.

The recent research on Slovakia’s national testing program provides a potential model for containing infectious disease outbreaks.

Clinical laboratories should be aware of the potential for quantitative differences in COVID-19 antibody levels, as demand for antibody testing may increase.

As new vaccine breakthrough research continues, Virginia Tech and UVA virologists develop innovative COVID-19 vaccine production platform.
The emergence of multiple SARS-CoV-2 variants brings fast-moving questions for clinical laboratory administrators about the ability to identify these new variants. A new mutation panel may offer an alternative to viral genome sequencing for indicating the possible presence of these variants.

The new research indicating a time-dependent result for COVID-19 testing may have significant implications for the clinical laboratories.

Use of this technology could be preventive, administered to people before they have an infection, says Rensselaer professor.

A sustained drop in COVID-19 diagnostic testing could present the clinical laboratory industry with opportunities, but it could also lead to potential damages if not handled proactively and correctly.

How the state plans to address potential waste of COVID-19 tests.
This new SARS-CoV-2 study carries risks and controversy.
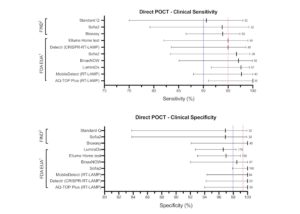
Findings of new research that focuses on the performance of rapid COVID-19 antigen and antibody tests.

Intermountain Healthcare Laboratories credit “adaptiveness” to achieving COVID-19 test milestone.

Joel Lefferts, PhD, of the Geisel School of Medicine at Dartmouth explains three major impacts that SARS-CoV-2 variants may have on testing in clinical laboratories.

With fast, affordable, easy-to-use home testing evidently a top priority, the incoming administration continues to adjust national response to the pandemic.

Findings of a new study published in the CDC’s Morbidity and Mortality Weekly Report (MMWR) that focused on the performance and public utility of Abbott BinaxNOW SARS-CoV-2 antigen test.

Insights into University of Illinois’ COVID-19 testing program using saliva test offer perspective on COVID-19 testing for universities.

Summary of new research into viral load with SARS-CoV-2.
HHS details state-level support for COVID-19 testing and monitoring, with the more than $19 billion to be allocated through the existing CDC ELC cooperative agreement.

While diagnostic testing for COVID-19 slowly shifts away from clinical laboratories, these changes can be beneficial.

Two key trends are explained as potential opportunities for clinical laboratories in the year ahead.

How SARS-CoV-2 (COVID-19) testing evolved from diagnostic testing only in clinical laboratories to three categories of primary diagnostic and secondary testing.

The scientific community starred in the development of several innovations designed to overcome the healthcare challenges brought on by the COVID-19 pandemic.

While face masks are thought to play an important role in mitigating the epidemiological risks of COVID-19, new research aims to understand how well various types of cloth masks and even “nonmedical” surgical masks work.

First EUA for at-home and over-the-counter COVID-19 testing removes the need for clinical laboratories to perform COVID-19 diagnostic testing.
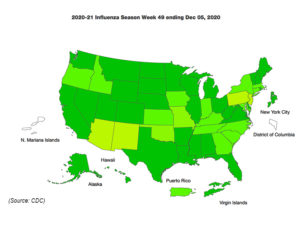
Week-by-week flu surveillance data show influenza testing in clinical laboratories compared to public health laboratories. While flu cases may be down, testing appears up.

The COVID-19 testing calculator tool developed by NIBIB is a mathematical model-based calculator that produces customized scenarios for COVID-19 surveillance testing.

While the availability of a nonprescription, at-home COVID-19 test collection kit will certainly increase the ease with which testing can be obtained, this new EUA seems to benefit LabCorp exclusively.

More on a recent study of 67 routine clinical laboratory tests that sought to identify COVID-19 biomarkers.
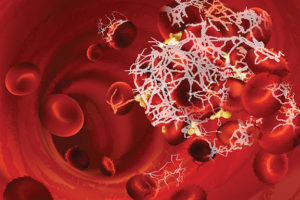
The cause of hypercoagulopathy with COVID-19 may be due to autoantibodies, new study shows, creating potential for increased demand for autoantibody testing.

The CDC’s Advisory Committee on Immunization Practices met Dec. 3, 2020, to determine COVID-19 vaccine allocation. Clinical laboratories may be among first to receive.

How at-home COVID-19 testing now authorized under FDA emergency use authorization and new tests coming to market may affect clinical laboratories.

New guidance positions FDA and now the NCI in process for those opting to use LDTs in their laboratories with or without FDA premarket review or authorization.

HHS Cue pilot program creates hybrid COVID-19 testing strategy, potentially fast tracks Cue into institutions and communities outside of five-state rollout.

Nov. 2, 2020, marks the third and final wave of rapid COVID-19 testing device distribution (to about 1,360 nursing homes), according to CMS. Chilly nursing home response may create opportunities for clinical labs.

Predictive tool for COVID-19 highlights the advantages of coupling machine learning with laboratory data.

How clinical laboratories can respond to a black market for fake negative COVID-19 test results.

Three strategies that benefited the community’s contact tracing program.

Essential guidance on legal, risk management, regulatory, and compliance issues for clinical laboratories and employers that are partnering for employee COVID-19 testing.

Two factors influencing demand for COVID-19 testing during flu season, according to recent analysis.

Interview with Mick Raich of Vachette Pathology on evolving payer and reimbursement rules and resolving billing and reimbursement difficulties for COVID-19 testing.

The Netherlands recently developed a national structure to ensure systematic distribution of SARS-CoV-2 testing and supplies for significantly expanding COVID-19 testing capacity. In the US, wide use of these antigen tests presents two key opportunities for clinical laboratories.

A particular grouping of several blood tests is needed to utilize this disease severity score. The predictive value of such clinical laboratory testing as a risk assessment model could drive an increase in seven key lab tests.

Three types of clinical laboratory testing support were stipulated with first FDA approval of antiviral drug for COVID-19 treatment.

Some 122 clinical laboratories reveal how supply shortages extend beyond COVID-19 testing supplies.

Possible new source of partnership for clinical laboratories for COVID-19 testing, especially in established networks.

Cost points and challenges emerging from new airport COVID-19 testing models.

Proactive steps for healthcare providers, such as clinical laboratories, to prevent a new HHS penalty related to the CARES Act funding they received.

Key points from three major US airlines offering COVID-19 testing as a way to spur air travel in US.

Highlights of a Wayne State University study that seeks to shed light on how viral load measurements are evolving with SARS-CoV-2 and the potential impact on mortality.
Five critical questions that must be answered in order to refine SARS-CoV-2 airborne transmission guidance.

Interview with Michael F. Wells, PhD, on growth of a national network of volunteer scientists to assist with COVID-19 clinical laboratory testing and more.

The reversal of the asymptomatic testing guidelines may benefit clinical laboratories from a business standpoint moving forward.

Interview with Matthew J. Binnicker, PhD, on the potential negatives of resolving staffing shortages by using research scientists for clinical laboratory testing.

Four significant findings of a Kaiser Health News state antigen test survey.

Clinical laboratory leaders need to be aware of situations where COVID-19 antigen tests may be inadequate.

Points of interest from new UCLA-University of Washington research attempting to trace hospital patients’ symptoms to earlier than thought COVID-19 cases.

Continued coronavirus research will provide more robust data about the efficacy of CRISPR-based technology as a new diagnostic testing method.

Now an FDA EUA for Verily’s SARS-CoV-2 pooled testing that can test 12 samples at once—Google sister company’s clinical laboratory test leanings continue entry into market.

Mayo Clinic Director of Clinical Virology Matthew J. Binnicker, PhD, explains when clinical laboratories should consider pooled testing for SARS-CoV-2.

To clear up any ambiguity, the EUA further clarifies that only one testing option is permitted under the initial EUA.

If the COVID-19 pandemic continues into 2021, then clinical laboratories can expect many new competitors for SARS-CoV-2 testing.

Federal collaboration with Abbott materializes into contract quickly after BinaxNOW COVID-19 Ag Card EUA announcement.

Pathologist Frederick (Fritz) Kiechle, MD, PhD, reviews two potential testing strategies associated with COVID-19 management.

With another change to CDC COVID-19 testing guidance, clinical laboratories may begin to see more fluctuations in test volumes because of potentially conflicting federal, state, and local testing guidance.
In an exclusive interview, pathologist Frederick (Fritz) Kiechle, MD, PhD, reviews biomarker changes and related clinical laboratory testing at different disease stages of COVID-19.
Joel Lefferts, PhD, of the Geisel School of Medicine at Dartmouth and Clinical Genomics and Advanced Technology (CGAT) at Dartmouth-Hitchcock Medical Center (DHMC), explains how SARS-CoV-2 virus genome sequencing and tracking the variants will complement contact tracing.

CDC continues to change SARS-CoV-2 diagnostic testing guidance.

What the open source protocol means to COVID-19 testing laboratories.

What happened with Ohio Gov. Mike DeWine’s coronavirus test.

In an exclusive interview, Dr. Joel Lefferts, a genomics specialist at Dartmouth’s Geisel School of Medicine, discusses coronavirus research around sequencing a small subset of the SARS-CoV-2 virus.

Whole slide images, annotations, and metadata in the digital pathology repository will be used as a reference data set for education, research, and future clinical trials aimed at limiting further infection, disease, and death.

A look at substantial state and federal efforts to scale point-of-care rapid antigen testing for SARS-CoV-2 coronavirus.

In an exclusive interview, Joshua Hayden, PhD, DABCC, FAACC, of Norton Healthcare, discusses COVID-19 associated coagulopathy, PE, and a potential clinical laboratory testing strategy for COVID-19 positive follow-ups.

Three points of impact included in new CDC quarantine guidelines, that will likely affect clinical laboratory PCR test volume.

As new testing options become available, the trend of diagnostic tests moving outside of clinical laboratory settings may soon begin to impact the volume of these tests.

Exploring a new disease detection method for COVID-19 in the UK.

New nursing home guidelines by CMS now require COVID-19 testing for nursing home staff in states with infection rates of 5% or more.

While the antigen test initiative of HHS will play a role in reducing the spread of COVID-19 among vulnerable populations and surrounding communities, mass rapid point of care testing will have implications for clinical laboratories.

While feces are not thought to transmit SARS-CoV-2, there are genetic materials found in the feces of infected individuals, that can be detected by PCR testing.

Screening asymptomatic people regardless of known or unknown COVID-19 exposure is useful, but it will be important to ensure that this testing is used appropriately and that contingency plans are in place to modify operations based on test results.

New research casts doubt on the evidence that SARS-CoV-2 serology testing yields useful data.

With serological assays for SARS-CoV-2 widely available, uncertainty remains around the accuracy of these tests. The revocation of Chembio’s SARS-CoV-2 serology EUA could indicate broader and stricter enforcement by the FDA.

What members of Congress wrote in response to federal departments’ interpretation and guidance of health insurers’ COVID-19 testing payment obligations.

The National Healthcare Safety Network (NHSN) module was one of four pathways to reporting crucial COVID-19 data to HHS. The NHSN module was deactivated with the TeleTracking HHS Protect portal becoming the primary pathway for hospitals, hospital laboratories, and other healthcare facilities. The decision has met criticism.

Overview of what CVS Health announced and described as a comprehensive, customizable COVID-19 testing solution for employers and universities.

Large diagnostic clinical laboratories publicly share pain of surge in COVID-19 test demand. Recent lab pro letter urges “visibility into the process of supply allocation.” Smaller labs may be getting shortchanged.

Employee screening tools being developed by technology companies create unique opportunity for clinical laboratories to pursue strategic partnerships.
Points of interest in the Harvard analysis of observations in Wuhan, China, as early as August 2019, that share new insights into the origins of the COVID-19 pandemic.

What the US Equal Employment Opportunity Commission (EEOC) updated in its June 17 guidance to employers on COVID-19 testing.
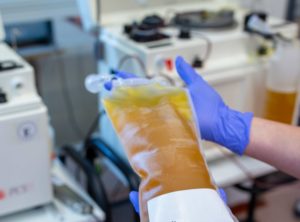
An interview with Mayo Clinic’s Elitza Theel, PhD, presents an overview of the state of convalescent plasma treatment for COVID-19.

Explanation of preanalytical issues with SARS-CoV-2 (COVID-19) testing in clinical laboratories and what to expect in the event of a second wave of infections in the fall.

At-home SARS-CoV-2 warning letters and implications for clinical laboratories and pathologists.

Overview of complaints around COVID-19 test payment expectations and attempts to reduce health insurers’ legislative and regulatory requirements.

In an exclusive interview, Frederick (Fritz) Kiechle, MD, PhD, explains how and why saliva has emerged as a sample type for detecting SARS-CoV-2, the coronavirus that causes COVID-19.

Pearls from the 90-minute educational webinar, “Achieving High Confidence Levels in the Quality and Accuracy of Your Clinical Lab’s Chosen COVID-19 Serology Tests,” presented June 11 and featuring James O. Westgard, PhD, and Sten Westgard.

In an exclusive interview, Danielle Sloane of Bass, Berry and Sims, discusses potentially tricky reimbursement for SARS-CoV-2 asymptomatic testing.
Latest on SARS-CoV-2 serology tests that have been removed or withdrawn from the FDA’s notification list, plus FDA’s notes on performance of available tests.

Impact of lower outpatient testing volumes on clinical laboratory and anatomic pathology services: Is there a bright side?

Explanation of HR 7025 and HR 7026 for clinical laboratories, hospitals, primary care practices, and other healthcare providers.

Influential quotes by Gary Procop, MD, MS, of the Cleveland Clinic, on the role of clinical laboratory and anatomic pathology group leaders in developing a national COVID-19 testing strategy.

Implications of blurring the lines between human testing laboratories and veterinarian labs.
With this recently approved testing paradigm that has emerged within the last few weeks, a significant question remains.

Though questions may remain about the reach and risks of the digital technologies used for COVID-19 contact tracing systems, clinical laboratory directors and pathologists will need to watch for changes in test demand.

Topics include adopting a platform that already has an FDA EUA, validating rapid test kits for use at remote sites, and other coronavirus antibody cross reactivity.
Some COVID-19 serology testing methods are demonstrating inadequate performance, according to Sten Westgard, who explained study data during a May 21 webinar. Learn how to assess fast-moving developments.

Mayo Clinic Laboratories is examining if utilization of SARS-CoV-2 serology testing may be optimized when coupled with PCR testing. Dr. Matthew J. Binnicker explains the lab test utilization strategy being evaluated to develop risk profiles and increase risk awareness.

An exclusive interview with Richard S. Cooper of the firm McDonald Hopkins addresses two categories of legal risks that have become evident for clinical laboratories with COVID-19 testing.

Repeat SARS-CoV-2 testing has been an issue that many clinical laboratories and pathology groups have encountered, making it a potential lab test utilization issue.

In mid-March, it was critical to provide regulatory flexibility for serology test developers. However, two major issues have surged through public and political discourse.
There’s good news coming for clinical laboratories as a tidal wave of orders for COVID-19 serology tests head their way while healthcare policymakers call upon
Crowdsourced data during COVID-19 pandemic has implications for clinical laboratories and pathology groups, including possibly predicting test volume.

What we know about the CDC’s SPHERES’ (SARS-CoV-2 Sequencing for Public Health Emergency Response, Epidemiology, and Surveillance) objectives.

Christopher Zavala of Northwell Health Labs explains use of Northwell’s mobile collections services app for blood draws, before and during the COVID-19 pandemic.

Roger D. Klein, MD, JD, explains how federal regulation around clinical laboratory developed tests (LDTs) has evolved during the COVID-19 pandemic.
Understand how the anti-kickback law connects to the Erik Santos COVID-19 test bribe case, plus EKRA.
One supply chain expert has several recommendations on how hospitals and clinical laboratories can respond to improve their access to needed supplies.
Review three briefs about first COVID-19 antibody studies in three cities, and what these studies suggest about demand for serology testing.

Explanation and discussion of federal guideline for daily clinical laboratory COVID-19 test reporting to FEMA.
Understand changes clinical laboratories and anatomic pathology groups are experiencing in testing volumes as a result of the COVID-19 pandemic.

An exclusive interview and podcast with Frederick (Fritz) Kiechle, MD, PhD, explores what actions clinical laboratories can take to implement reliable antibody tests and benefit from potential serology testing reimbursement.

Brain imaging research would seem to indicate that the lasting effects of COVID-19 brain changes could be permanent.

What University of Southern California research showed about suppression of cells’ mitochondria in COVID-19 immune system response.

Bitcoin becomes a potential method of payment for COVID-19 testing for point-of-care tests performed by one Florida laboratory.

Recent shift in perspective and openness to theories about SARS-CoV-2 origins mean that the truth behind the COVID-19 pandemic will eventually be discovered.

New findings about an innovative method for COVID-19 diagnostic testing that is said to provide instant results.

COVID-19 breakthrough shows that the virus uses three different techniques to evade the immune system and increase its viral load in infected individuals.
Now available for streaming: How does your lab begin to prepare itself to succeed in a post-pandemic world? Start your plan now to navigate the transition. Attend this complimentary webinar and hear the ways in which your lab’s current testing can create assets for the future, and how you can best position your lab for success in a post-pandemic environment by leveraging your diagnostic data as a strategic asset.
Available for free streaming: Learn the important clinical implications (epidemiological, prognostic, and predictive) of new COVID-19 “variants of concern,” the importance of early detection of these variants, and steps your lab can take to confidently detect the COVID-19 virus with a very high degree of specificity and sensitivity, given new variants.
Gain a clear understanding of when it makes sense to implement pooled testing, specifically for COVID-19, understand the potential benefits of pooled testing, as well as learn the important features to look for when selecting pooled testing workflow technology.

Learn how the right suite of applications can empower your lab through technology, help you tailor your outreach program to your lab clients, and as a result improve market share with an efficient and effective outreach strategy.

Take away from this webinar a clear understanding of how your lab can rapidly ramp up its COVID-19 testing, offer the right support for the right tests (RT-PCR, serology) and effectively serve physician customers, while maintaining adherence to security and compliance requirements.

Now that your clinical laboratory has decided on which COVID-19 serology test it will buy and offer to physicians, patients, and employers with wellness screening programs, your lab team must succeed with the challenging process of validation. This is required—not just for your lab to meet CLIA requirements—but for your lab team to have high confidence that the COVID-19 serology test results are accurate, reproducible, and appropriate for use in patient care. Join us for this program as James O. Westgard and Sten Westgard, lead you through the process.

Brain imaging research would seem to indicate that the lasting effects of COVID-19 brain changes could be permanent.

What University of Southern California research showed about suppression of cells’ mitochondria in COVID-19 immune system response.

Bitcoin becomes a potential method of payment for COVID-19 testing for point-of-care tests performed by one Florida laboratory.

Recent shift in perspective and openness to theories about SARS-CoV-2 origins mean that the truth behind the COVID-19 pandemic will eventually be discovered.

New findings about an innovative method for COVID-19 diagnostic testing that is said to provide instant results.

COVID-19 breakthrough shows that the virus uses three different techniques to evade the immune system and increase its viral load in infected individuals.